Using Agar Slants - In Pictures
- gregorach
- Under the Table
- Posts: 1912
- Joined: Wed Jun 08, 2011 10:07 am
- Location: Edinburgh
- Contact:
Re: Using Agar Slants - In Pictures
That's a bloody good question barney, and the honest answer is that I don't know. I suspect that lack of oxygen has some part in it, but personally I chill them once they reach a level of growth that I'm happy with - I suspect they could grow further, and could well spread over the entire surface of the slant eventually if you gave them long enough. Another thing to remember is that yeast can't move, so the colony can only expand by adding new cells around the edge. As colony grows and the edge gets longer, expansion slows down because it needs ever more cells.
Cheers
Dunc
Dunc
-
barney
Re: Using Agar Slants - In Pictures
The reason I ask Dunc is that never have the amount of coverage shown on the photos of slopes earlier in the thread. Mine always seem to plateaux well before then and I chill them down when it looks like the party is over. I have just switched to slopes in glass test tubes 6" long and I have more growth than I ever got with with the small plastic tubes and that's only after 2 days. I am hopeful that I may get somewhere near complete coverage. I just wondered if it was down to larger air volume in the tube and subsequently more available oxygen.
- gregorach
- Under the Table
- Posts: 1912
- Joined: Wed Jun 08, 2011 10:07 am
- Location: Edinburgh
- Contact:
Re: Using Agar Slants - In Pictures
It also depends on how much yeast you inoculate with in the first place, and how well spread out it it.
Cheers
Dunc
Dunc
-
Lars
Re: Using Agar Slants - In Pictures
Guys, I have been slanting for under a year now. I've some strains that I've only kept one slant of and I'll be wanting more. How do I reslant from an old slant?
1. Do I simply run loop across old slant and streak new slant?
2. Does original slant need to be at room temp to restreak?
3. Or do I need to make a starter wort or dilute existing slant with sterile water and reinnoculate usign that solution?
Thanks
L
1. Do I simply run loop across old slant and streak new slant?
2. Does original slant need to be at room temp to restreak?
3. Or do I need to make a starter wort or dilute existing slant with sterile water and reinnoculate usign that solution?
Thanks
L
-
Twistedfinger
- Hollow Legs
- Posts: 366
- Joined: Wed Nov 10, 2010 10:35 pm
- Location: From Darlo living in Bolton and feeling damp !
Re: Using Agar Slants - In Pictures
I've done it both ways, making a starter has more risk of contamination but is useful if there is a doubt about viability. Taking just a loop off an existing slant and re-streaking is easy and quick. Plus you can "pick" your colony if the slant allows you to, but this depends on how well it was streaked in the first place.
Why not try both, take a loop sample and make a starter with the rest ?
Why not try both, take a loop sample and make a starter with the rest ?
- gregorach
- Under the Table
- Posts: 1912
- Joined: Wed Jun 08, 2011 10:07 am
- Location: Edinburgh
- Contact:
Re: Using Agar Slants - In Pictures
The "proper" way to do it is to streak a plate from your slant, allow the colonies to grow for a few days, and they inoculate your new slants from selected individual colonies. If you're not going to go to that length, then there's really no point in doing anything more than a straight slant-to-slant transfer - there's no point in making up a starter, it's just one more stage to potentially introduce contamination. And no, you don't need to warm the original slant back to room temp first.
Cheers
Dunc
Dunc
-
Lars
Re: Using Agar Slants - In Pictures
Great, thats actually the answer I hoped for, simple slant to slant with no messing around...gregorach wrote:The "proper" way to do it is to streak a plate from your slant, allow the colonies to grow for a few days, and they inoculate your new slants from selected individual colonies. If you're not going to go to that length, then there's really no point in doing anything more than a straight slant-to-slant transfer - there's no point in making up a starter, it's just one more stage to potentially introduce contamination. And no, you don't need to warm the original slant back to room temp first.
-
YeastWhisperer
Re: Using Agar Slants - In Pictures
[quote="Wolfy"]If I had followed my own instructions as outlined above, I'd not have had this problem, but yeast is not the only thing that likes living on the wort/agar media, so one must be pedantic and careful about sanitation and storage at all points of the process:
[img]http://i184.photobucket.com/albums/x37/ ... _yeast.jpg[/img]
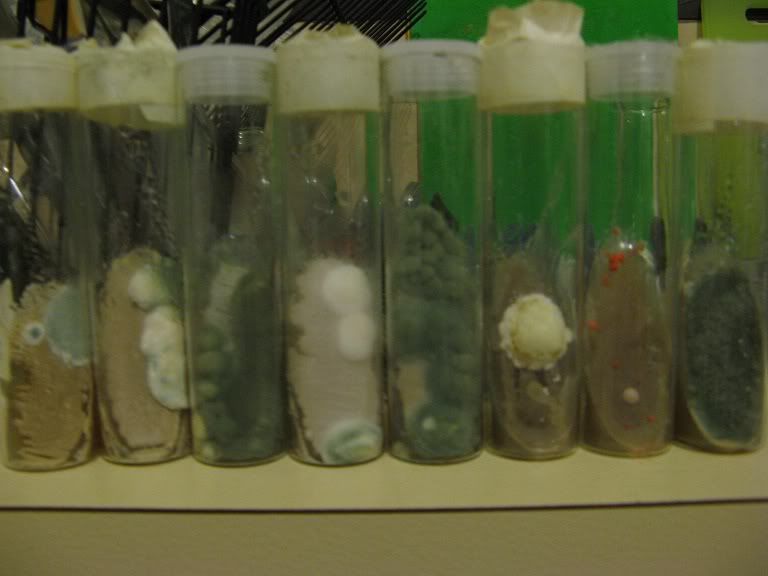
With the 4 slants on the left, the green mould seems to have grown (inside a sealed zip-lock bag, inside the fridge) on both sides of the slightly damp masking tape, which allowed the condensation inside the tube to contaminated the wort/agar media. The three slants on the left were most likely contaminated when they were made and the growths are result a tiny infection having a year to grow. Screw-top tubes sealed with Parafilm, and more pedantic sanitary procedures when making the slants, should have prevented these problems, so I edited the first post to more clearly explain that.
Also to note - in the picture - is that on the 5 month old slants (on the left) the remaining yeast still looks fairly healthy and viable, however the yeast on the year old slants (on the right) does not appear to be as healthy and is quite dried out.
Luckily the contaminated slants represent less than 10% of the total number I have stored, and there was also a second uncontaminated slant (and frozen sample) of each yeast strain stored. [/quote]
[/quote]
One should always plate single colonies from liquid culture before transferring a colony or two to an uninoculated slant. Here's something that I recently wrote on hombrewtalk.com:
While the process requires attention to detail, plating yeast is not difficult (a petri dish partially filled with solidified wort or another culturing medium is known as a "plate"). I plated my first yeast culture after brewing my third or fourth batch of beer. However, that was back in 1993 when the state of commercial yeast in the amateur brewing community was fairly dismal.
Plating requires extreme attention to sanitation. I make my own plates and slants from 5 degrees Plato (1.020 SG) hopped wort and agar (a gelatin that is made from seaweed that remains solid at room temperature). Unlike starters, plating and slanting gelatinized wort should be autoclaved to render it absolutely sterile. While most microflora are killed at temperatures above 150F, one has to take the temperate up to 250F for at least 13 minutes in order to kill spores. This process is accomplished using an autoclave (I usually autoclave plating and slanting media for 15 minutes). An autoclave is little more than a laboratory-grade pressure cooker. One can use a normal pressure cooker as an autoclave in a home setting. I know that some amateur brewers only boil their plating and slanting gelatinized wort, but I do not recommend that practice. If one wants to produce plates and slants that will reliably keep for months in a refrigerator before being used, one should autoclave one's plating and slanting media. A small pressure cooker can be purchased fairly cheaply.
Plating is usually performed within the confines of a laminar flow hood in a laboratory setting. The air inside of a laminar flow hood is under positive pressure to keep anything from settling onto the plates (the air source is filtered).
A plate being streaked in a laminar flow hood
[img]http://www.dnr.sc.gov/ael/facilities/hood-mrri.jpg[/img]
The tool that is in the right hand of the scientist shown above is known as an inoculation loop. A disposable inoculation loop is being used in the photograph. I use a reusable loop that is made from nichrome wire. A reusable loop has to be heat sterilized using a Bunsen burner or an alcohol lamp before being used (the loop is heated in the flame until it glows). An alcohol lamp is much more practical for a home-based lab because denatured alcohol is nowhere near as explosive as propane.
A slightly bent reusable nichrome wire loop
[img]http://upload.wikimedia.org/wikipedia/c ... n_loop.JPG[/img]
In a home setting, one can plate over a clean, well-sanitized surface using only an alcohol lamp or Bunsen burner, as the air around the burner is rising. However, all transfers need to be performed within a few inches of the flame.
The basic yeast plating process is known as a four quadrant streak. The process is started by heating the loop until it glows. The loop is then dipped into the yeast culture to be plated, which will immediately cool it to the temperature of the culture. One then lightly draws a zigzag pattern on one quarter (quadrant) of the plate with the tip of the loop. The loop is then heated until it glows and cooled on a spot on the plate that has not yet been inoculated (I leave a small area in the middle of the plate untouched to use for cooling the loop). The plate is rotated ninety degrees, and the next quadrant is streaked by overlapping one's zigzag pattern with the pattern in the first quadrant. The third quadrant is streaked by once again heating the loop until it glows, cooling it on an untouched area of the plate, and overlapping one's zigzag pattern with the second quadrant. The process is repeated with the fourth quadrant; however, one must be careful to only overlap the third quadrant with one's zigzag pattern. The plate is then covered and allowed to incubate at room temperature (I store the plate in a clean well-sanitized container while it is incubating). What one is doing with this process is diluting the culture.
After incubating the plate at room temperature for a couple of days, the first quadrant of the plate will usually have a solid line of microflora where one drew the zigzag pattern on the plate. The zigzag pattern will start to have gaps in it in the second quadrant. By the time that one gets to the fourth quadrant, there will be well separated individual dots. These dots are referred to as "colonies." Each colony is composed of the offspring of a single yeast cell.
An incubated four quadrant-streaked plate
[img]http://www.microbelibrary.org/images/up ... k_fig6.jpg[/img]
Yeast colonies are fairly easy to identify from other microflora. They are creamy white in color and round in shape. Anything that is fuzzy or non-creamy white is a nasty that one does not want in one's beer. When selecting a yeast colony for propagation, one should select only colonies that exhibit good morphology, that is, colonies that have a nice round shape. Non-round colonies tend to be genetic mutants. One wants to pick an average size colony to transfer to a slant. A slant is a screw-cap culture tube that contains the same gelatinized wort as one's plates. The culture tube is positioned at angle while the gelatinized wort is cooling; hence, the name "slant."
Screw-cap culture tube
[img] http://www.cardinal.com/us/en/distribut ... 360-1A.jpg[/img]
A slant is inoculated using the same sterile technique that was used to streak the plate. The loop is heated until it glows and cooled on an area of the plate that contains no visible microflora growth (I often use the same area of the plate that I used when streaking it). A yeast colony is then scooped from the plate using the loop and streaked onto the slant (just smear it on the surface of the slant). The culture tube is capped and allowed to incubate at room temperate for a few days before being stored on the refrigerator. The slant contains what is known as a "single-cell pure culture" because it was grown from a single yeast cell.
A properly grown starter from a slant will be significantly purer that anything that one can acquire commercially in the homebrew trade. I brewed for ten years using only home lab isolated and propagated yeast. My beers were significantly cleaner tasting than those of my peers who used commercially-produced yeast. My batch-to-batch quality control was better as well. Yeast is the most important ingredient in brewing; therefore, it should receive the most attention in the brewing process.
[img]http://i184.photobucket.com/albums/x37/ ... _yeast.jpg[/img]
With the 4 slants on the left, the green mould seems to have grown (inside a sealed zip-lock bag, inside the fridge) on both sides of the slightly damp masking tape, which allowed the condensation inside the tube to contaminated the wort/agar media. The three slants on the left were most likely contaminated when they were made and the growths are result a tiny infection having a year to grow. Screw-top tubes sealed with Parafilm, and more pedantic sanitary procedures when making the slants, should have prevented these problems, so I edited the first post to more clearly explain that.
Also to note - in the picture - is that on the 5 month old slants (on the left) the remaining yeast still looks fairly healthy and viable, however the yeast on the year old slants (on the right) does not appear to be as healthy and is quite dried out.
Luckily the contaminated slants represent less than 10% of the total number I have stored, and there was also a second uncontaminated slant (and frozen sample) of each yeast strain stored.
One should always plate single colonies from liquid culture before transferring a colony or two to an uninoculated slant. Here's something that I recently wrote on hombrewtalk.com:
While the process requires attention to detail, plating yeast is not difficult (a petri dish partially filled with solidified wort or another culturing medium is known as a "plate"). I plated my first yeast culture after brewing my third or fourth batch of beer. However, that was back in 1993 when the state of commercial yeast in the amateur brewing community was fairly dismal.
Plating requires extreme attention to sanitation. I make my own plates and slants from 5 degrees Plato (1.020 SG) hopped wort and agar (a gelatin that is made from seaweed that remains solid at room temperature). Unlike starters, plating and slanting gelatinized wort should be autoclaved to render it absolutely sterile. While most microflora are killed at temperatures above 150F, one has to take the temperate up to 250F for at least 13 minutes in order to kill spores. This process is accomplished using an autoclave (I usually autoclave plating and slanting media for 15 minutes). An autoclave is little more than a laboratory-grade pressure cooker. One can use a normal pressure cooker as an autoclave in a home setting. I know that some amateur brewers only boil their plating and slanting gelatinized wort, but I do not recommend that practice. If one wants to produce plates and slants that will reliably keep for months in a refrigerator before being used, one should autoclave one's plating and slanting media. A small pressure cooker can be purchased fairly cheaply.
Plating is usually performed within the confines of a laminar flow hood in a laboratory setting. The air inside of a laminar flow hood is under positive pressure to keep anything from settling onto the plates (the air source is filtered).
A plate being streaked in a laminar flow hood
[img]http://www.dnr.sc.gov/ael/facilities/hood-mrri.jpg[/img]
The tool that is in the right hand of the scientist shown above is known as an inoculation loop. A disposable inoculation loop is being used in the photograph. I use a reusable loop that is made from nichrome wire. A reusable loop has to be heat sterilized using a Bunsen burner or an alcohol lamp before being used (the loop is heated in the flame until it glows). An alcohol lamp is much more practical for a home-based lab because denatured alcohol is nowhere near as explosive as propane.
A slightly bent reusable nichrome wire loop
[img]http://upload.wikimedia.org/wikipedia/c ... n_loop.JPG[/img]
In a home setting, one can plate over a clean, well-sanitized surface using only an alcohol lamp or Bunsen burner, as the air around the burner is rising. However, all transfers need to be performed within a few inches of the flame.
The basic yeast plating process is known as a four quadrant streak. The process is started by heating the loop until it glows. The loop is then dipped into the yeast culture to be plated, which will immediately cool it to the temperature of the culture. One then lightly draws a zigzag pattern on one quarter (quadrant) of the plate with the tip of the loop. The loop is then heated until it glows and cooled on a spot on the plate that has not yet been inoculated (I leave a small area in the middle of the plate untouched to use for cooling the loop). The plate is rotated ninety degrees, and the next quadrant is streaked by overlapping one's zigzag pattern with the pattern in the first quadrant. The third quadrant is streaked by once again heating the loop until it glows, cooling it on an untouched area of the plate, and overlapping one's zigzag pattern with the second quadrant. The process is repeated with the fourth quadrant; however, one must be careful to only overlap the third quadrant with one's zigzag pattern. The plate is then covered and allowed to incubate at room temperature (I store the plate in a clean well-sanitized container while it is incubating). What one is doing with this process is diluting the culture.
After incubating the plate at room temperature for a couple of days, the first quadrant of the plate will usually have a solid line of microflora where one drew the zigzag pattern on the plate. The zigzag pattern will start to have gaps in it in the second quadrant. By the time that one gets to the fourth quadrant, there will be well separated individual dots. These dots are referred to as "colonies." Each colony is composed of the offspring of a single yeast cell.
An incubated four quadrant-streaked plate
[img]http://www.microbelibrary.org/images/up ... k_fig6.jpg[/img]
Yeast colonies are fairly easy to identify from other microflora. They are creamy white in color and round in shape. Anything that is fuzzy or non-creamy white is a nasty that one does not want in one's beer. When selecting a yeast colony for propagation, one should select only colonies that exhibit good morphology, that is, colonies that have a nice round shape. Non-round colonies tend to be genetic mutants. One wants to pick an average size colony to transfer to a slant. A slant is a screw-cap culture tube that contains the same gelatinized wort as one's plates. The culture tube is positioned at angle while the gelatinized wort is cooling; hence, the name "slant."
Screw-cap culture tube
[img] http://www.cardinal.com/us/en/distribut ... 360-1A.jpg[/img]
A slant is inoculated using the same sterile technique that was used to streak the plate. The loop is heated until it glows and cooled on an area of the plate that contains no visible microflora growth (I often use the same area of the plate that I used when streaking it). A yeast colony is then scooped from the plate using the loop and streaked onto the slant (just smear it on the surface of the slant). The culture tube is capped and allowed to incubate at room temperate for a few days before being stored on the refrigerator. The slant contains what is known as a "single-cell pure culture" because it was grown from a single yeast cell.
A properly grown starter from a slant will be significantly purer that anything that one can acquire commercially in the homebrew trade. I brewed for ten years using only home lab isolated and propagated yeast. My beers were significantly cleaner tasting than those of my peers who used commercially-produced yeast. My batch-to-batch quality control was better as well. Yeast is the most important ingredient in brewing; therefore, it should receive the most attention in the brewing process.
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Using Agar Slants - In Pictures
YeastWhisperer wrote:One should always plate single colonies from liquid culture before transferring a colony or two to an uninoculated slant. Here's something that I recently wrote on hombrewtalk.com:Wolfy wrote:If I had followed my own instructions as outlined above, I'd not have had this problem, but yeast is not the only thing that likes living on the wort/agar media, so one must be pedantic and careful about sanitation and storage at all points of the process:
With the 4 slants on the left, the green mould seems to have grown (inside a sealed zip-lock bag, inside the fridge) on both sides of the slightly damp masking tape, which allowed the condensation inside the tube to contaminated the wort/agar media. The three slants on the left were most likely contaminated when they were made and the growths are result a tiny infection having a year to grow. Screw-top tubes sealed with Parafilm, and more pedantic sanitary procedures when making the slants, should have prevented these problems, so I edited the first post to more clearly explain that.
Also to note - in the picture - is that on the 5 month old slants (on the left) the remaining yeast still looks fairly healthy and viable, however the yeast on the year old slants (on the right) does not appear to be as healthy and is quite dried out.
Luckily the contaminated slants represent less than 10% of the total number I have stored, and there was also a second uncontaminated slant (and frozen sample) of each yeast strain stored.
While the process requires attention to detail, plating yeast is not difficult (a petri dish partially filled with solidified wort or another culturing medium is known as a "plate"). I plated my first yeast culture after brewing my third or fourth batch of beer. However, that was back in 1993 when the state of commercial yeast in the amateur brewing community was fairly dismal.
Plating requires extreme attention to sanitation. I make my own plates and slants from 5 degrees Plato (1.020 SG) hopped wort and agar (a gelatin that is made from seaweed that remains solid at room temperature). Unlike starters, plating and slanting gelatinized wort should be autoclaved to render it absolutely sterile. While most microflora are killed at temperatures above 150F, one has to take the temperate up to 250F for at least 13 minutes in order to kill spores. This process is accomplished using an autoclave (I usually autoclave plating and slanting media for 15 minutes). An autoclave is little more than a laboratory-grade pressure cooker. One can use a normal pressure cooker as an autoclave in a home setting. I know that some amateur brewers only boil their plating and slanting gelatinized wort, but I do not recommend that practice. If one wants to produce plates and slants that will reliably keep for months in a refrigerator before being used, one should autoclave one's plating and slanting media. A small pressure cooker can be purchased fairly cheaply.
Plating is usually performed within the confines of a laminar flow hood in a laboratory setting. The air inside of a laminar flow hood is under positive pressure to keep anything from settling onto the plates (the air source is filtered).
A plate being streaked in a laminar flow hood
The tool that is in the right hand of the scientist shown above is known as an inoculation loop. A disposable inoculation loop is being used in the photograph. I use a reusable loop that is made from nichrome wire. A reusable loop has to be heat sterilized using a Bunsen burner or an alcohol lamp before being used (the loop is heated in the flame until it glows). An alcohol lamp is much more practical for a home-based lab because denatured alcohol is nowhere near as explosive as propane.
A slightly bent reusable nichrome wire loop
In a home setting, one can plate over a clean, well-sanitized surface using only an alcohol lamp or Bunsen burner, as the air around the burner is rising. However, all transfers need to be performed within a few inches of the flame.
The basic yeast plating process is known as a four quadrant streak. The process is started by heating the loop until it glows. The loop is then dipped into the yeast culture to be plated, which will immediately cool it to the temperature of the culture. One then lightly draws a zigzag pattern on one quarter (quadrant) of the plate with the tip of the loop. The loop is then heated until it glows and cooled on a spot on the plate that has not yet been inoculated (I leave a small area in the middle of the plate untouched to use for cooling the loop). The plate is rotated ninety degrees, and the next quadrant is streaked by overlapping one's zigzag pattern with the pattern in the first quadrant. The third quadrant is streaked by once again heating the loop until it glows, cooling it on an untouched area of the plate, and overlapping one's zigzag pattern with the second quadrant. The process is repeated with the fourth quadrant; however, one must be careful to only overlap the third quadrant with one's zigzag pattern. The plate is then covered and allowed to incubate at room temperature (I store the plate in a clean well-sanitized container while it is incubating). What one is doing with this process is diluting the culture.
After incubating the plate at room temperature for a couple of days, the first quadrant of the plate will usually have a solid line of microflora where one drew the zigzag pattern on the plate. The zigzag pattern will start to have gaps in it in the second quadrant. By the time that one gets to the fourth quadrant, there will be well separated individual dots. These dots are referred to as "colonies." Each colony is composed of the offspring of a single yeast cell.
An incubated four quadrant-streaked plate
Yeast colonies are fairly easy to identify from other microflora. They are creamy white in color and round in shape. Anything that is fuzzy or non-creamy white is a nasty that one does not want in one's beer. When selecting a yeast colony for propagation, one should select only colonies that exhibit good morphology, that is, colonies that have a nice round shape. Non-round colonies tend to be genetic mutants. One wants to pick an average size colony to transfer to a slant. A slant is a screw-cap culture tube that contains the same gelatinized wort as one's plates. The culture tube is positioned at angle while the gelatinized wort is cooling; hence, the name "slant."
Screw-cap culture tube
A slant is inoculated using the same sterile technique that was used to streak the plate. The loop is heated until it glows and cooled on an area of the plate that contains no visible microflora growth (I often use the same area of the plate that I used when streaking it). A yeast colony is then scooped from the plate using the loop and streaked onto the slant (just smear it on the surface of the slant). The culture tube is capped and allowed to incubate at room temperate for a few days before being stored on the refrigerator. The slant contains what is known as a "single-cell pure culture" because it was grown from a single yeast cell.
A properly grown starter from a slant will be significantly purer that anything that one can acquire commercially in the homebrew trade. I brewed for ten years using only home lab isolated and propagated yeast. My beers were significantly cleaner tasting than those of my peers who used commercially-produced yeast. My batch-to-batch quality control was better as well. Yeast is the most important ingredient in brewing; therefore, it should receive the most attention in the brewing process.
Your post count is too low to post pictures so I have replied in order for them to be seen. Good post and good news to have another experienced yeast rancher on the forum. Particularly pleased by your last comment, you will find many brewers who have a rather casual approach to it
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
YeastWhisperer
Re: Using Agar Slants - In Pictures
[quote="orlando"]Your post count is too low to post pictures so I have replied in order for them to be seen. Good post and good news to have another experienced yeast rancher on the forum. Particularly pleased by your last comment, you will find many brewers who have a rather casual approach to it  .[/quote]
.[/quote]
Thanks for re-posting my posting. I knew that my inability to use BBcode had to have something to do with passing an initiation ritual.
Thanks for re-posting my posting. I knew that my inability to use BBcode had to have something to do with passing an initiation ritual.
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Using Agar Slants - In Pictures
No, you've got that to comeYeastWhisperer wrote: I knew that my inability to use BBcode had to have something to do with passing an initiation ritual.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer