Testing my water
-
Chrissyr63
- Piss Artist
- Posts: 153
- Joined: Sat Apr 06, 2013 9:46 pm
- Location: Hounslow (and Barnstable at weekends)
Testing my water
Hi All,
after reading all the water treatment threads that are about at the moment I bought myself one of the Salifert KH\Alk testers.
Did this on my water in Devon and it seemed to come out as 0.96ml left (just 3 drops and it was pink) so alk of 0.10 meq\l - I did a test against the supplied solution and that did come out as set so think I was doing it right.
Is this water too soft? Tend to brew Wheat and darker ales at the moment - should I be adding in something.
Think I need to read through some more of those threads again.
Am going to test my London water next week and assume that will be much higher
C
after reading all the water treatment threads that are about at the moment I bought myself one of the Salifert KH\Alk testers.
Did this on my water in Devon and it seemed to come out as 0.96ml left (just 3 drops and it was pink) so alk of 0.10 meq\l - I did a test against the supplied solution and that did come out as set so think I was doing it right.
Is this water too soft? Tend to brew Wheat and darker ales at the moment - should I be adding in something.
Think I need to read through some more of those threads again.
Am going to test my London water next week and assume that will be much higher
C
-
Charles1968
Re: Testing my water
You might need to add chalk, gypsum or calcium chloride. Try and find out more about the levels of the various ions in your water. If you can't find the info online, you can send a sample to Murphys. Ditto your London water
If you set up both water profiles on brewers friend, you'll be able to blend them, which should give you really good control (if you're happy to transport water between devon and London). Brewersfriend will tell you what salts you need to get close to target.
If you set up both water profiles on brewers friend, you'll be able to blend them, which should give you really good control (if you're happy to transport water between devon and London). Brewersfriend will tell you what salts you need to get close to target.
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: Testing my water
For stouts porters and anything containing a reasonable amount of crystal malt, you need to be looking at raising your alkalinity for your mash liquor only. Chalk is often suggested, but even in the mash it doesn't dissolve a great deal and so has limited use. Better options would be potassium hydrogen carbonate or sodium hydrogen carbonate, of these two the potassium hydrogen carbonate could be considered the better choice, as the level of potassium in finished beer is high anyway (it comes from the malt). Ideally you don't want to increase sodium content above 50ppm.
You could also consider adding a tsp of gypsum and or calcium chloride (or a mixture of the two) to the mash and another to the boil (for a 25L batch of beer) use more gypsum if you want a hoppy drier beer, and calcium chloride if you want a malty beer.
For your wheat beers you can probably get away with just adding a tsp of gypsum to the mash and another to the boil.
I must admit I like Charles suggestion of blending your water, I have been known to do this in the past, bringing 5 gallons of water back with me from my In laws in suffolk, or from a private well at Ribblehead in Yorkshire, and then brew with a mix of my relatively low mineral content water, and the high mineral content water. I wasn't as technical as Charles suggests, I just simply mixed it to get the alkalinity in the ball park (fine tued it with acid afterwards), and accepted where the other ions went, water treatment is not a subject where you need a high degree of accuracy, a PhD in analytical chemistry and a particle accelerator to 'get it right' There or there abouts is more than adequate for the vast majority of beer styles.
If you really know what you have in your water send a PM to the user wallybrew on here, he offers a reliable, and accurate water testing service through Phoenix analytical.
You could also consider adding a tsp of gypsum and or calcium chloride (or a mixture of the two) to the mash and another to the boil (for a 25L batch of beer) use more gypsum if you want a hoppy drier beer, and calcium chloride if you want a malty beer.
For your wheat beers you can probably get away with just adding a tsp of gypsum to the mash and another to the boil.
I must admit I like Charles suggestion of blending your water, I have been known to do this in the past, bringing 5 gallons of water back with me from my In laws in suffolk, or from a private well at Ribblehead in Yorkshire, and then brew with a mix of my relatively low mineral content water, and the high mineral content water. I wasn't as technical as Charles suggests, I just simply mixed it to get the alkalinity in the ball park (fine tued it with acid afterwards), and accepted where the other ions went, water treatment is not a subject where you need a high degree of accuracy, a PhD in analytical chemistry and a particle accelerator to 'get it right' There or there abouts is more than adequate for the vast majority of beer styles.
If you really know what you have in your water send a PM to the user wallybrew on here, he offers a reliable, and accurate water testing service through Phoenix analytical.
-
Charles1968
Re: Testing my water
I reckon 50:50 Devon to London water would probably be a great all-rounder. To make a wild guess, mix 75% Devon to 25% London for the wheat beers or lagers and maybe 75% London and 25% Devon for dark stuff.Aleman wrote:I just simply mixed it to get the alkalinity in the ball park (fine tued it with acid afterwards), and accepted where the other ions went
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: Testing my water
Agreed, certainly better that trying to use either as it isCharles1968 wrote:I reckon 50:50 Devon to London water would probably be a great all-rounder. To make a wild guess, mix 75% Devon to 25% London for the wheat beers or lagers and maybe 75% London and 25% Devon for dark stuff.Aleman wrote:I just simply mixed it to get the alkalinity in the ball park (fine tuned it with acid afterwards), and accepted where the other ions went
-
Chrissyr63
- Piss Artist
- Posts: 153
- Joined: Sat Apr 06, 2013 9:46 pm
- Location: Hounslow (and Barnstable at weekends)
Re: Testing my water
Hi Charlie and Aleman,
thanks for the replies - I was just thinking of dipping my toe into the water treatment ocean with the alk kit but was a bit surprised the Devon water was so low (am expecting the London one to be high with the amount of scale we get in the kettle (for tea)).
Missus was confused when I bought the aquarium kit (we haven't got any fish!) can't imagine what she will think when I load 20lt of water from London before we leave!
I brewed a saison this afternoon and put a couple of teaspoons of gypsum into the mash water so will see how that goes.
Do remember the first time I did the Seymour citra gold I followed his recipe and added a bit of gypsum and loved the beer. Second time I didn't and it tasted less hoppy - might try again with the addition.
C
thanks for the replies - I was just thinking of dipping my toe into the water treatment ocean with the alk kit but was a bit surprised the Devon water was so low (am expecting the London one to be high with the amount of scale we get in the kettle (for tea)).
Missus was confused when I bought the aquarium kit (we haven't got any fish!) can't imagine what she will think when I load 20lt of water from London before we leave!
I brewed a saison this afternoon and put a couple of teaspoons of gypsum into the mash water so will see how that goes.
Do remember the first time I did the Seymour citra gold I followed his recipe and added a bit of gypsum and loved the beer. Second time I didn't and it tasted less hoppy - might try again with the addition.
C
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Testing my water
Carbon dioxide from the atmosphere is absorbed by rain and forms carbonic acid. This acidic water reacts with calcium (or magnesium) deposits of limestones and chalks to create alkalinity. While what we today know as Devon, Corwall too, was once covered by limestone, aeons of wind and rain erroded most of it leaving insoluble rock, so not only is alkalinity low in your water, there is little else either. Accordingly added calcium salt will improve the brewing environment for the vast majority of beer styles. Mashing malt in stable and favourable conditions relies upon matching the buffering properties of alkalinity which that water does not have in sufficient quantity for darker malts. That does not mean it won't unaltered make a drinkable dark beer, just that it will produce its own style and temperament and to explore a fuller range of more established darker beer it will be necessary to add alkalinity as well as calcium salts.
London is built above chalk, slowly sinking by the persistent southward movement of the land to its north. The course of the River Thames is greatly south of its original course and the basin filled with clay. Water from wells used by early London brewers varied substantially, dependent upon whether water was drawn from clay, chalk or other strata and if it contained any brackish river water. It will be interesting to know what alkalinity you find with your Salifert kit and how it compares with that below which I think was 260mg/l CaCO3.
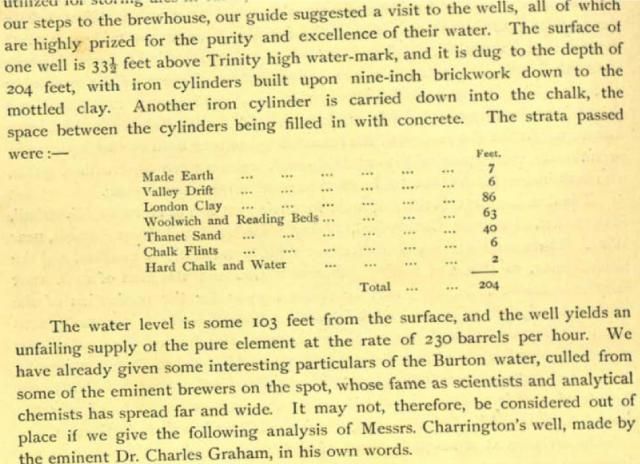
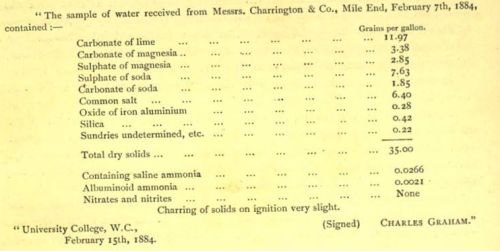
London is built above chalk, slowly sinking by the persistent southward movement of the land to its north. The course of the River Thames is greatly south of its original course and the basin filled with clay. Water from wells used by early London brewers varied substantially, dependent upon whether water was drawn from clay, chalk or other strata and if it contained any brackish river water. It will be interesting to know what alkalinity you find with your Salifert kit and how it compares with that below which I think was 260mg/l CaCO3.


Without patience, life becomes difficult and the sooner it's finished, the better.
-
BenB
Re: Testing my water
I'm in North London and fed by the Neasden reservoir. We have a fairly stable alkalinity of 210-220 mg/l CaCO3. I test each time I brew and in between times if I'm bored  and it really doesn't change that much. I just downloaded the new water report and is pretty similar to last time. Max change 10÷ for the ions.
and it really doesn't change that much. I just downloaded the new water report and is pretty similar to last time. Max change 10÷ for the ions.
Re: Testing my water
Should the alkalinity of sparge water also be adjusted?Aleman wrote:For stouts porters and anything containing a reasonable amount of crystal malt, you need to be looking at raising your alkalinity for your mash liquor only.
Maidstone Brewers Homebrew Meets - Next Meet 14:00 Wednesday 27 December
https://Twitter.com/maidstonebrews https://www.facebook.com/groups/maidstonebrewers
https://Twitter.com/maidstonebrews https://www.facebook.com/groups/maidstonebrewers
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Testing my water
Yes, in fact your sparge water should be treated to circa 25-30 ppm for all brews. Mash liquour needs to be treated acording to style/grist composition.legion wrote:Should the alkalinity of sparge water also be adjusted?Aleman wrote:For stouts porters and anything containing a reasonable amount of crystal malt, you need to be looking at raising your alkalinity for your mash liquor only.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: Testing my water
Actually for the OP, then NO he doesn't need to do anything to the sparge liquor as the alkalinity is already may lower that 25-30.orlando wrote:Yes, in fact your sparge water should be treated to circa 25-30 ppm for all brews. Mash liquour needs to be treated acording to style/grist composition.legion wrote:Should the alkalinity of sparge water also be adjusted?Aleman wrote:For stouts porters and anything containing a reasonable amount of crystal malt, you need to be looking at raising your alkalinity for your mash liquor only.
Orlando is correct though in that you adjust alkalinity to that required for the style (colour) of beer you are making, and the sparge water needs to alkalinity to be around 25-30 (alternatively add acid until the pH is around 6.0, to prevent extraction of tanins) . .. Personally I'd bet the two methods would produce the same results anyway
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Testing my water
If I could taste the difference blindfolded I would be like a dog with two dic.......Aleman wrote: Personally I'd bet the two methods would produce the same results anyway
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Chrissyr63
- Piss Artist
- Posts: 153
- Joined: Sat Apr 06, 2013 9:46 pm
- Location: Hounslow (and Barnstable at weekends)
Re: Testing my water
Hi all,
the style recommendation was the bit I wasn't too sure about - for darker ales it is better to be nearer 75+ so I should increase (or use my London water).
Also didn't realise that sparge water could be a different (lower) level (I assume because you are just rinsing the grains by that stage so the ph isn't as important).
Learn more on here every day.
Chris
the style recommendation was the bit I wasn't too sure about - for darker ales it is better to be nearer 75+ so I should increase (or use my London water).
Also didn't realise that sparge water could be a different (lower) level (I assume because you are just rinsing the grains by that stage so the ph isn't as important).
Learn more on here every day.
Chris
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Testing my water
Chrissyr63 wrote: assume because you are just rinsing the grains by that stage so the ph isn't as important).
Learn more on here every day.
pH is always important if you want to brew first class beer. What isn't so important is whereabouts in the ideal range you are.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Testing my water
As sugars are washed from the grains, their buffering power diminishes and sparge liquors containing high levels of alkalinity will cause the pH to rise, hence the reason to consider lower alkalinity in sparge water.Chrissyr63 wrote:Hi all,
the style recommendation was the bit I wasn't too sure about - for darker ales it is better to be nearer 75+ so I should increase (or use my London water).
Also didn't realise that sparge water could be a different (lower) level (I assume because you are just rinsing the grains by that stage so the ph isn't as important).
Learn more on here every day.
Chris
Problems you might have if the level of calcium is insufficient quanty will also include high mash and sparge pH. While both calcium and alkalinity have major influences on pH, Calcium is also a vital building block in the vast array of processes in brewing. Unless you know what level of calcium is present or you can measure the pH of your mash and runnings it would be prudent to consider adding calcium salts to the mash and maybe your sparge water.
Without patience, life becomes difficult and the sooner it's finished, the better.

